Bsc Classification

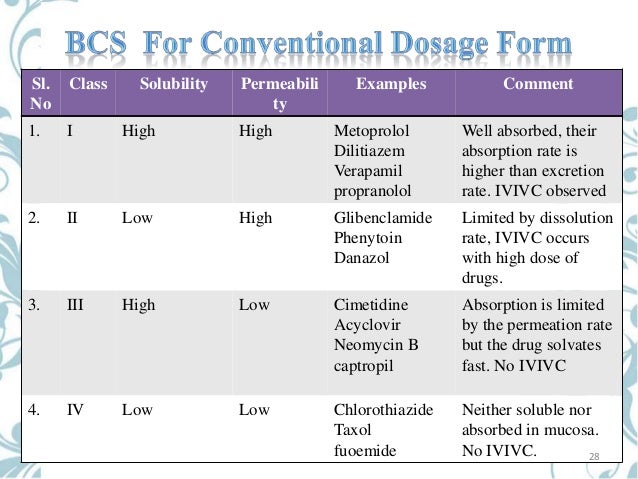
Fda Bcs Classification
To provide sponsors of new drug submissions with the information necessary to comply with Division 8 of the Food and Drug Regulations ( Regulations) with respect to Biopharmaceutics Classification System (BCS) based biowaivers for comparative bioavailability studies to be used in support of the safety and efficacy of a drug. This information is applicable to all submission types where comparative bioavailability studies would normally provide pivotal evidence in support of the safety and efficacy of a product. In vivo human data collected for the purpose of submission to Health Canada should be collected in accordance with generally accepted clinical practices that are designed to ensure the protection of the rights, safety and well-being of subjects. They should be collected in compliance with the good clinical practices referred to in Division 5 of the Regulations and described in the International Conference on Harmonisation (ICH) Guidance (Topic E6) on Good Clinical Practice. The principles of Good Manufacturing Practice as indicated in Part C, Division 2 of the Regulations should be adhered to wherever applicable. Virtua tennis 4 download. Absorption - the uptake of substance from a solution into or across tissues.